Breaking News
New pain pill approved in U.S. designed to cut risk of addiction – National
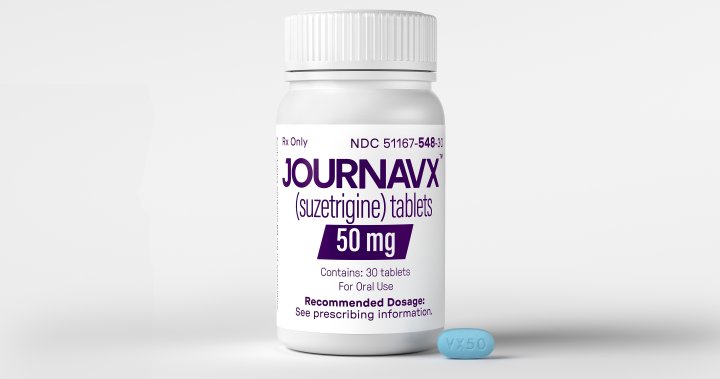
The U.S. Food and Drug Administration has approved a new type of pain pill that aims to eliminate the risks of addiction and overdose associated with opioid medications like Vicodin and OxyContin.
The FDA approved Vertex Pharmaceuticals’ Journavx for short-term pain following surgery or injuries, marking the first new pharmaceutical approach to pain treatment in over 20 years. This new medication provides an alternative to opioids and over-the-counter drugs like ibuprofen and acetaminophen. However, its effectiveness is modest, and the lengthy development process highlights the challenges of pain management innovation.
Studies on over 870 patients with acute pain from foot and abdominal surgeries showed that Vertex’s drug offered more relief than a placebo but did not surpass a common opioid-acetaminophen combination pill.
Dr. Michael Schuh of the Mayo Clinic, a pharmacist and pain medicine expert not involved in the research, commented, “It’s not a slam dunk on effectiveness, but it is a slam dunk in that it’s a very different pathway and mechanism of action. So, I think that shows a lot of promise.”
The new drug will be priced at $15.50 per pill, significantly more expensive than generic opioids available for $1 or less.

Vertex initiated research on the drug in the 2000s amid a surge in opioid overdoses, primarily due to excessive prescribing of opioid painkillers for common conditions like arthritis and back pain. While prescriptions have declined in the past decade, the current opioid epidemic is driven by illicit fentanyl rather than pharmaceuticals.

Get weekly health news
Receive the latest medical news and health information delivered to you every Sunday.
Opioids work by binding to brain receptors that receive nerve signals from the body, leading to both pain relief and addictive effects. In contrast, Vertex’s drug functions by blocking proteins that initiate pain signals sent to the brain.
“In developing medicines without the addictive risks of opioids, a key focus is on blocking pain signaling before it reaches the brain,” explained Dr. David Altshuler of Vertex to The Associated Press.
Common side effects reported with the drug include nausea, constipation, itching, rash, and headache.
Dr. Charles Argoff of the Albany Medical Center, who advised Vertex on the drug’s development, stated, “The new medication has side effect profiles that are inherently different and do not involve the risk of substance abuse and other key side effects associated with opioids.”

The idea to target pain-signaling proteins originated from research on individuals with a rare hereditary condition that causes insensitivity to pain.
Vertex has garnered attention for its extensive drug pipeline, aiming to obtain FDA approval for multiple drugs covering various forms of chronic pain, which present a larger financial opportunity than acute pain.
However, Vertex’s stock price dropped in December following disappointing results in a mid-stage study on chronic nerve pain in the lower back and legs. The drug did not show significant improvement over a placebo.
Biotechnology analyst Brian Abrahams expressed concerns in a research note that the results posed a significant challenge to Vertex’s pipeline estimates, potentially impacting its value across multiple pain forms.
Despite this setback, Vertex executives plan to proceed with a new late-stage study on the drug, believing that a different trial design could yield better outcomes and lead to FDA approval for chronic pain.
© 2025 The Canadian Press
-

 Breaking News2 years ago
Breaking News2 years agoCroatia to reintroduce compulsory military draft as regional tensions soar
-

 Destination1 year ago
Destination1 year agoSingapore Airlines CEO set to join board of Air India, BA News, BA
-

 Gadgets1 year ago
Gadgets1 year agoSupernatural Season 16 Revival News, Cast, Plot and Release Date
-

 Productivity2 years ago
Productivity2 years agoHow Your Contact Center Can Become A Customer Engagement Center
-

 Tech News2 years ago
Tech News2 years agoBangladeshi police agents accused of selling citizens’ personal information on Telegram
-

 Gadgets10 months ago
Gadgets10 months agoGoogle Pixel 9 Pro vs Samsung Galaxy S25 Ultra: Camera Comparison Review
-

 Gaming2 years ago
Gaming2 years agoThe Criterion Collection announces November 2024 releases, Seven Samurai 4K and more
-

 Gadgets10 months ago
Gadgets10 months agoFallout Season 2 Potential Release Date, Cast, Plot and News