Breaking News
Pennsylvania woman Juanita Gantt claims Ozempic nearly killed her
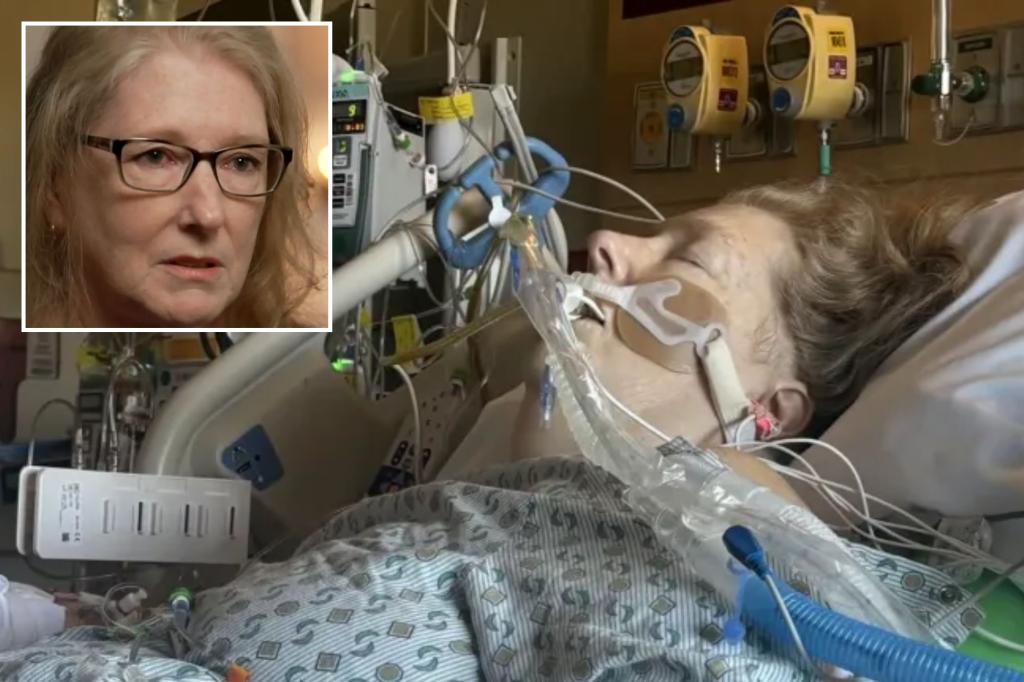
A mother from Pennsylvania is taking legal action against the manufacturer of Ozempic and Wegovy, alleging that she almost lost her life due to the prescription drugs and was not adequately warned about the potential severe side effects.
Juanita Gantt stated that her doctor prescribed these popular weight-loss medications to her as she was at a higher risk for diabetes and wanted to lose more than 20 pounds.
Initially prescribed Wegovy, Gantt was later switched to Ozempic. Both drugs are GLP-1 (glucagon-like peptide-1) medications produced by Novo Nordisk.
She felt fine during the first few months of treatment until her husband found her unconscious on the floor in October 2023.
Speaking in an interview on CBS News, Gantt expressed her shock at not being warned about the possible side effects that led to her severe health issues.
After parts of her large intestine died and had to be removed, Gantt suffered a cardiac arrest during her recovery from surgery.
Concerned about Gantt’s life, health officials even had to inform her daughter about the critical situation.
Heartbroken by the situation, Gantt shared her sorrow about her daughter receiving such distressing news.
Due to the removal of her colon, Gantt now has to carry an ileostomy bag wherever she goes.
The traumatic experience led Gantt to file a lawsuit against Novo Nordisk, claiming that the warning labels on their drugs do not adequately caution users and doctors about serious side effects like gastroparesis, stomach paralysis, or bowel obstruction.
In response to the allegations, the prescription drugmaker stated that the lawsuits are baseless and they will vigorously defend against them.
Originally developed for diabetics to stimulate insulin release and reduce blood sugar levels, Ozempic and Wegovy have seen a surge in usage for weight loss in recent years.
Ozempic’s website lists side effects such as pancreatitis, vision changes, low blood sugar, kidney problems, severe allergic reactions, gallbladder issues, and more.
In March 2024, Wegovy became the first weight loss medication approved to help prevent cardiovascular events in adults with cardiovascular disease and obesity or overweight, as per the U.S. Food and Drug Administration.
While Ozempic is not FDA approved for weight management, it is approved for treating type 2 diabetes. However, due to its popularity for weight loss, doctors have been prescribing it off-label in recent years.
According to Gallup’s analysis, approximately 15 million Americans have used GLP-1 drugs for weight loss, with a majority of users being over the age of 40.
-

 Breaking News2 years ago
Breaking News2 years agoCroatia to reintroduce compulsory military draft as regional tensions soar
-

 Destination1 year ago
Destination1 year agoSingapore Airlines CEO set to join board of Air India, BA News, BA
-

 Gadgets1 year ago
Gadgets1 year agoSupernatural Season 16 Revival News, Cast, Plot and Release Date
-

 Productivity2 years ago
Productivity2 years agoHow Your Contact Center Can Become A Customer Engagement Center
-

 Tech News2 years ago
Tech News2 years agoBangladeshi police agents accused of selling citizens’ personal information on Telegram
-

 Gadgets10 months ago
Gadgets10 months agoGoogle Pixel 9 Pro vs Samsung Galaxy S25 Ultra: Camera Comparison Review
-

 Gaming2 years ago
Gaming2 years agoThe Criterion Collection announces November 2024 releases, Seven Samurai 4K and more
-

 Gadgets10 months ago
Gadgets10 months agoFallout Season 2 Potential Release Date, Cast, Plot and News